PTC124-GD-041-DMD
(Also known as study 041)
The clinical trial of ataluren in nonsense mutation Duchenne muscular dystrophy (NMDMD)
Study 041 is a new clinical trial for boys and young men who are living with nonsense mutation Duchenne Muscular Dystrophy (nmDMD).
Who Can Enter Study 041?
To ensure that participants who enroll in Study 041 get the most benefit, and to ensure their safety, there are strict criteria around who is able to enter the study. Your doctor will help to decide whether you/your child can take part.
Main Inclusion Criteria
- Documentation proving that you have undertaken a genetic test which shows you/your child has nmDMD.
- You/your child is male and five years of age or older.
- You/your child is able to walk at least 150 meters (165 yards) without help during a six-minute walking test.
- You/your child is able to undertake certain timed tests.
- You/your child has been on corticosteroid treatment (i.e. prednisone, prednisolone, or deflazacort) for at least 12 months immediately before the start of study treatment, with no significant change in dose within three months before the start of the study (other than for body weight change).
Trial Design
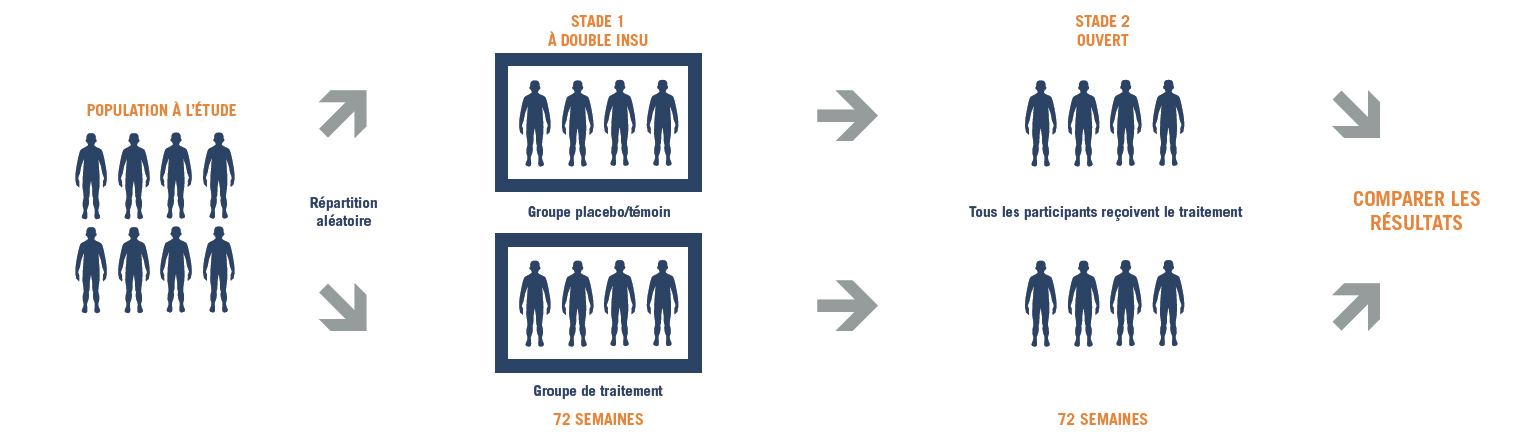
Stage One
Stage One of Study 041 is looking at how the investigational treatment, ataluren, affects the ability to walk and endurance in males aged five years and older with nmDMD, compared to placebo (a substance that looks and tastes the same as ataluren but does not actually contain the drug). The study will also look at the ability of participants to climb up and down stairs, walk/run certain distances, use their upper limbs and measure lung function. The safety of ataluren will also be assessed.
Stage Two
Stage Two of Study 041 will compare results in participants who started on ataluren from the beginning of the trial with those who started taking ataluren from the start of stage two. All participants will be involved in both stages of the trial.
If you/your child decides to enroll in Study 041, you may help drive a better understanding of nmDMD, as well as current and future treatments, which may benefit nmDMD patients in the future.
Frequently asked questions
What is ataluren?
Ataluren is an investigational therapy being studied for the treatment of people with nmDMD who are still able to walk and aged five years or older. It is administered three times per day, mixed with a liquid or semi-solid food and taken orally. People with nmDMD are unable to produce a protein, dystrophin, which helps keep muscles healthy. Without dystrophin, muscles become weaker over time. PTC Therapeutics is studying ataluren to determine if it helps the body produce dystrophin, which may help slow down muscle weakening.
Is ataluren safe?
Ataluren has been studied in over 1,000 patients in clinical trials. During these clinical trials, ataluren was found to be well-tolerated in patients with nmDMD and has demonstrated a favourable safety profile for this condition.
Will I need to pay anything to be involved in the trial?
No, all costs involved in the trial, including physical examinations, screening, laboratory and other tests, as well as the cost of the drug, will be covered by PTC Therapeutics, who is funding Study 041.
You may be reimbursed for all reasonable*costs for travel, meals and accommodation necessary for your visits to the study site the clinic, which will be approximately every 12 weeks for the first stage of the trial, and every 24 weeks for the second stage of the trial.
How will ataluren affect other treatments that I am taking?
If you/your child are/is taking any medications, then the doctor will advise you/your child of potential problems and discuss treatment options with you/your child. However, to participate in the trial you/your child must be taking treatments known as corticosteroids, and ataluren can be taken alongside these medications.
What happens if/when ataluren is approved in my country? Can I leave the trial?
If you/your child enroll in Study 041, we would like a commitment that you/your child will follow through with the study obligations, unless a doctor determines you/your child should discontinue for health or safety reasons. However, participation in this study is entirely voluntary, and if you wish to do so, you/your child are able to withdraw from the study at any point for any reason, or no reason.
What do I have to do to be involved in Study 041?
If you/your child would like to participate in the trial, before being enrolled, you/your child will need to discuss the trial with your doctor, and agree (consent) to being involved. You/your child will be asked to sign a consent form(s). The consent form(s) provides detailed information, which will outline the risks and benefits of participating in the trial. If you/your child have/has any questions about this information, talk to your doctor as it is important that you are fully informed. The consent form(s) also explains the basic facts about the trial in age-appropriate language. When you/your child signs these forms you will be given copies to keep.
The next step involves some checks to ensure that you/your child is eligible to participate in the trial. These checks, known as screening procedures, will include tests that are timed to assess your/your child’s ability to run/walk 10 meters, climb four stairs, descend four stairs, and stand up from lying down on your back within 30 seconds. These checks will take place in the two weeks before your/your child’s trial starts. A genotyping test, to ensure that you/your child has a nonsense mutation of the dystrophin gene, will be administered at baseline, or the start of the trial.
What do I have to do during Study 041?
If you/your child are/is accepted into the trial, you will be randomly assigned to either the ataluren group or the placebo group.
Ataluren or placebo is taken through the mouth. Every day for the duration of the trial, you/your child will need to prepare and take the medication or placebo three times a day: in the morning, at midday and in the evening.
Ideally, there should be about six hours between morning and midday doses, about six hours between midday and evening doses, and about 12 hours between evening doses and the morning dose on the next day.
For More Information
Email: patientinfo@ptcbio.com
Toll free: +1 (866) 282-5873
Ask for the original document: mcduberger@laforcedmd.com
Learn more
- Learn more about nonsense mutation: here
- Learn more about ataluren (Translarna™) at www.ptcbio.com
- Translarna™ (ataluren)
- Pioneers in DMD therapy